
Laverdia-CA1 2.5mg (50 tablets)
Laverdia®-CA1 is the first oral tablet FDA conditionally approved to treat lymphoma in dogs. The active ingredient in LAVERDIA-CA1 is verdinexor, a substance that works by preventing tumor suppressing proteins from leaving the nucleus of cells, resulting in disruption of cancer cell survival and eventual cancer cell death.
IMPORTANT SAFETY INFORMATION:
For use in dogs only. Laverdia®-CA1(verdinexor) is conditionally approved for the treatment of lymphoma in dogs. NOT FOR USE IN HUMANS. KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. CHILDREN SHOULD NOT COME INTO CONTACT WITH Laverdia®-CA1. Pregnant women, women who may become pregnant, nursing women and children should not handle or administer Laverdia®-CA1 or come into contact with the feces, urine, saliva, or vomit of treated dogs for 3 days following treatment.
Storage Instructions:
Store the bottles at controlled room temperature 20° to 25°C (68° – 77°F).
Prescription items are NON-RETURNABLE and NON-REFUNDABLE.
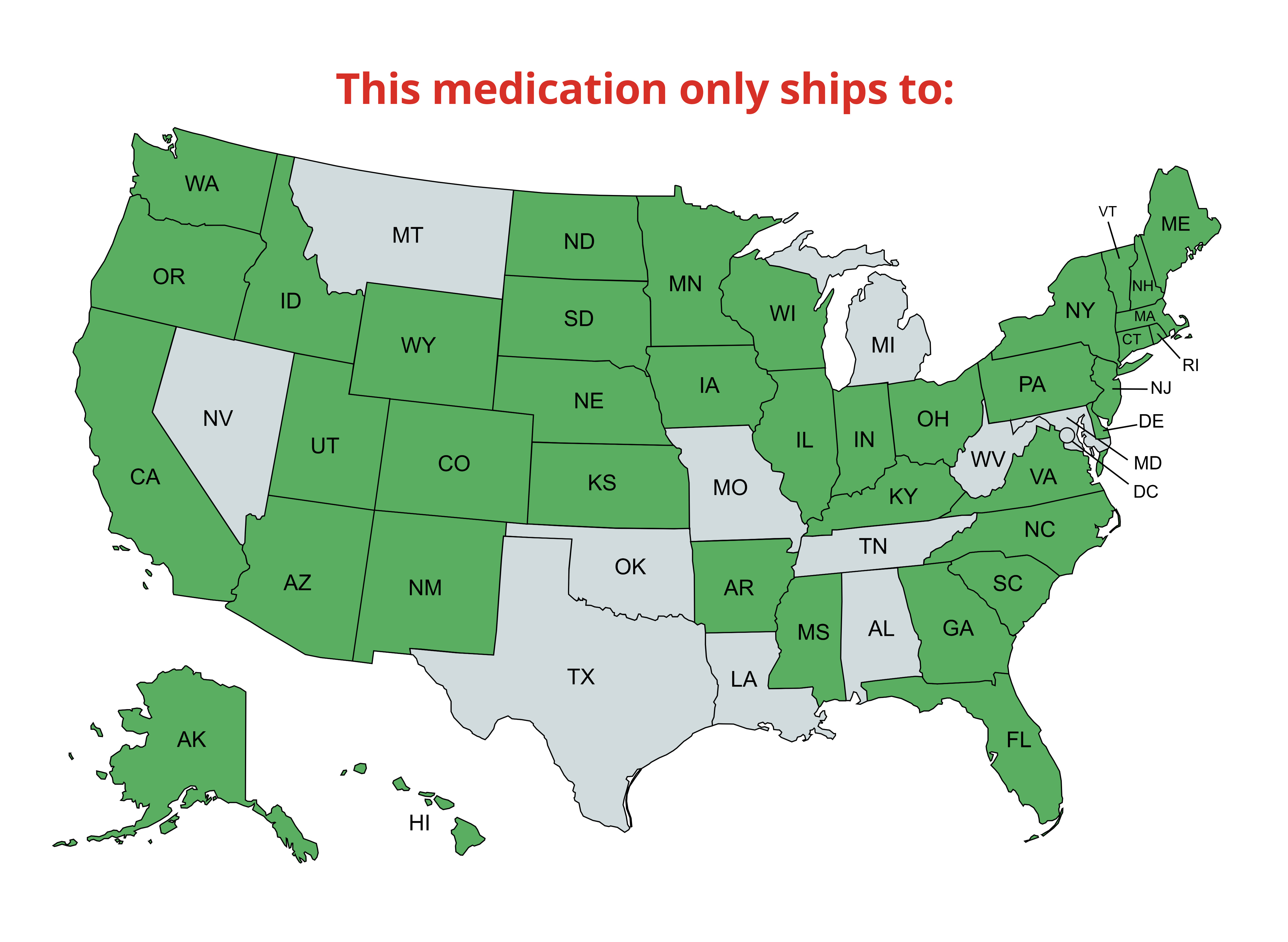
Recommended for the Laverdia-CA1 2.5 mg (50 tablets)
Product title
Vendor
£19.99 | £24.99
Product title
Vendor
£19.99 | £24.99
Product title
Vendor
£19.99 | £24.99
Product title
Vendor






