Atopica for Dogs
- Decreases itching and skin lesions by a large amount
- Successfully demonstrated to be beneficial for the majority of canines.
- Enables you to manage your pet's issue without using steroids
- It is safe to use for extended periods without the common serious side effects seen with steroids.
Prescription items are NON-RETURNABLE and NON-REFUNDABLE.
Please note product may arrive in a Hardy Paw Pharmacy vial, manufacturer packaging is shown for reference.
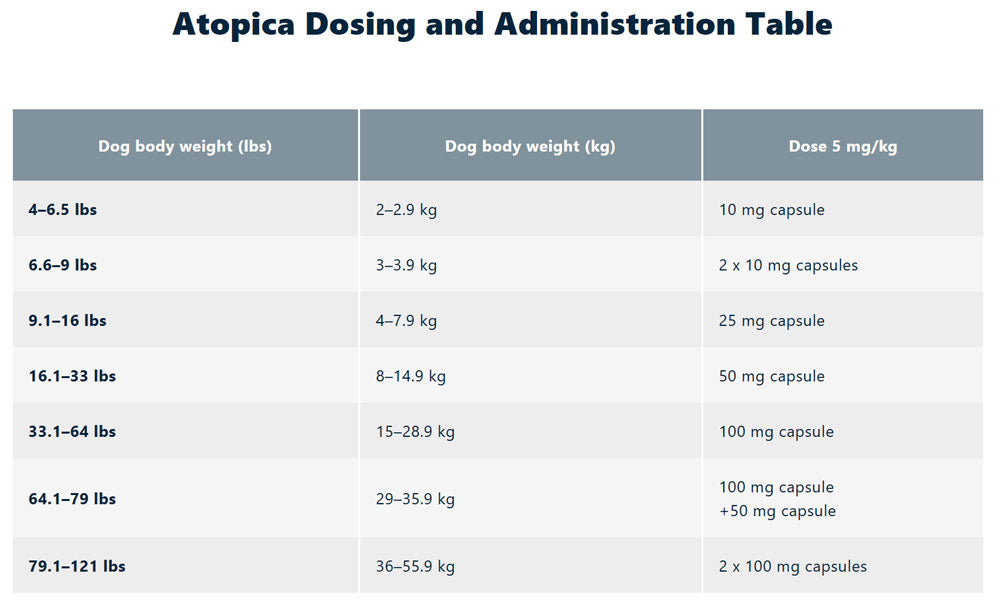
DOSAGE AND ADMINISTRATION:
The initial dose of ATOPICA is 5 mg/kg/day (3.3-6.7 mg/kg/day) as a single daily dose for 30 days. Following this initial daily treatment period, the dose of ATOPICA may be tapered by decreasing the frequency of dosing to every other day or twice weekly, until a minimum frequency is reached which will maintain the desired therapeutic effect. ATOPICA should be given at least one hour before or two hours after a meal. If a dose is missed, the next dose should be administered (without doubling) as soon as possible, but dosing should be no more frequent than once daily.
Contraindications:
ATOPICA is contraindicated for use in dogs with a history of neoplasia. Do not use in dogs with a hypersensitivity to cyclosporine.
Warnings:
ATOPICA (cyclosporine) is a systemic immunosuppressant that may increase the susceptibility to infection and the development of neoplasia.
Human Warnings:
Not for human use. Keep this and all drugs out of reach of children.
For use only in dogs.
Capsules should not be broken or opened. Wear gloves during administration. Wash hands after administration. In case of accidental ingestion, seek medical advice immediately and provide the package insert or the label to the physician.
Precautions:
The safety and effectiveness of ATOPICA has not been established in dogs less than 6 months of age or less than 4 lbs body weight. ATOPICA is not for use in breeding dogs, pregnant or lactating bitches.
As with any immunomodulation regimen, exacerbation of sub-clinical neoplastic and infectious conditions may occur.
Gastrointestinal problems and gingival hyperplasia may occur at the initial recommended dose (See Animal Safety).
ATOPICA may cause elevated levels of serum glucose, and should be used with caution in cases with diabetes mellitus. If signs of diabetes mellitus develop following the use of ATOPICA, consideration should be given to tapering or discontinuing the dose.
ATOPICA should be used with caution with drugs that affect the P-450 enzyme system. Simultaneous administration of ATOPICA with drugs that suppress the P-450 enzyme system, such as azoles (e.g. ketoconazole), may lead to increased plasma levels of cyclosporine.
Since the effect of cyclosporine use on dogs with compromised renal function has not been studied, ATOPICA should be used with caution in dogs with renal insufficiency.
There have been reports of convulsions in human adult and pediatric patients receiving cyclosporine, particularly in combination with high dose methylprednisolone (See Animal Safety).
Killed vaccines are recommended for dogs receiving ATOPICA because the impact of cyclosporine on the immune response to modified live vaccines is unknown (See Animal Safety).
Storage Conditions:
ATOPICA should be stored and dispensed in the original unit-dose container at controlled room temperature between 59° and 77°F (15-25°C).
FAQ
- What is the most important thing I should know about Atopica for Dogs?
- Notify your veterinarian immediately if your pet develops a fever (over 103°), painful urination, tiredness, sneezing, coughing, or runny nose. These symptoms could be early signs of dangerous side effects. Don't use in dogs with a history of neoplasia, with a hypersensitivity to cyclosporine, or in reproducing dogs. Use with caution in dogs with diabetes mellitus or renal insufficiency, and with drugs that affect the P-450 pathway. Killed vaccines are recommended. Atopica is a systemic immunosuppressant that may increase susceptibility to infection and development of neoplasia. See the product label for complete safety information.
- What is Atopica for Dogs?
- Atopica for Dogs (cyclosporine modified) is an immunosuppressant available by prescription. Atopica is FDA-approved for use in the treatment of atopic dermatitis in dogs at least 6 months of age weighing at least 4 lbs. Atopica for Dogs is available in 15 capsule blister packs in strengths of 10 mg for dogs 4-9 lbs, 25 mg for dogs 9.1-16 lbs, 50 mg for dogs 16.1-33 lbs and 100 mg for dogs 33.1-64 lbs. For dogs, 64.1-79 lbs, give a combination of one 100 mg capsule and one 50 mg capsule as a single dose. For dogs 79.1-121 lbs give, two 100 mg capsules as a single dose. Atopica for Dogs is given once a day to start until satisfactory improvement is seen, usually 4 to 8 weeks. The medication can then be given every other day until the clinical signs of atopic dermatitis are satisfactorily controlled, then the medication can be given every 3 or 4 days.
- What should I discuss with my veterinarian before giving Atopica for Dogs?
- Tell your veterinarian if your pet has liver disease, kidney disease, cancer, high blood pressure, a viral, bacterial, or fungal infection, or any other serious or chronic condition. Tell your veterinarian if your pet is pregnant or lactating, and if you plan to breed your pet.
- How should Atopica for Dogs be given?
- Give Atopica for Dogs exactly as directed by your veterinarian. If you do not understand these directions, ask your veterinarian or pharmacist to explain them to you. Give Atopica for Dogs 1 hour before or 2 hours after a meal. Allow plenty of water for the pet to drink. Do not remove a capsule from the blister pack until required for use. When the capsule is removed from the blister pack there is a characteristic, noticeable smell which is normal. Wear gloves during administration. Capsules should not be broken or opened. Wash hands after administration. Your veterinarian may want your pet to have regularly scheduled blood tests during treatment to monitor effectiveness and side effects.
- What are the potential side effects of Atopica for Dogs?
- If any of the following serious side effects occur, stop giving Atopica for Dogs to your pet and seek emergency veterinary medical attention: an allergic reaction (difficulty breathing, swelling of the lips, tongue, or face, and hives). Gastrointestinal problems and gingival hyperplasia may occur at the initial dose. Other less serious side effects may occur. Continue giving Atopica for Dogs and talk to your veterinarian if your pet develops vomiting, soft stools or diarrhea, muscle cramps, muscle weakness, loss of appetite, and change of hair coat. Other side effects may occur. Talk to your veterinarian about any side effect that seems unusual or bothersome to your pet.
- What happens if I miss giving a dose of Atopica to Dogs?
- Give the missed dose as soon as you remember. If it is almost time for the next dose, skip the dose you missed and give the next regularly scheduled dose. Do not give a double dose unless otherwise directed by your veterinarian.
- What happens if I overdose my pet on Atopica for Dogs?
- Seek emergency veterinary medical treatment.
- What should I avoid while giving Atopica for Dogs to my pet?
- The safe use in breeding, pregnant, or lactating pets has not been determined. Do not use Atopica for Dogs in pets with known allergies to the medication. The drug should not be used in pets with kidney disease, stomach ulcers, and certain blood disorders. Prolonged use of Atopica can result in bacterial or fungal infection related to a decreased effect on the immune system
- What other drugs will affect Atopica for Dogs?
- Tell your veterinarian what medications your pet is currently using and any new products, including herbal remedies you may start to give. Drug/drug interactions could cause a decrease in effectiveness or an increase in side effects of either Atopica or the other medication being given. Examples of medications that may cause drug/drug interactions are: SMZ-TMP (Bactrim, Septra), gentamicin, etodolac (EtoGesic), piroxicam (Feldene), ketoconazole (Nizoral), cimetidine (Tagamet), ranitidine (Zantac), itraconazole (Sporanox), methylprednisolone (Medrol), erythromycin, Allopurinol (Zyloprim), metoclopramide (Reglan), prednisolone, digoxin (Lanoxin), or any type of vaccination.
Recommended for the Atopica for Dogs - Cyclosporine Capsules 25mg
Product title
Vendor
19,99 lei | 24,99 lei
Product title
Vendor
19,99 lei | 24,99 lei
Product title
Vendor
19,99 lei | 24,99 lei
Product title
Vendor




















