Product title
Vendor
$19.99 | $24.99
Product title
Vendor
$19.99 | $24.99
Product title
Vendor
$19.99 | $24.99
Product title
Vendor
$19.99 | $24.99
Targeted reproductive support for cattle fertility
Product Description
CYSTORELIN® is an FDA-approved injectable reproductive solution for beef and dairy cattle designed to support the treatment of ovarian follicular cysts and help synchronize estrous cycles for fixed time artificial insemination (FTAI). It contains gonadorelin to promote natural hormone release that supports ovulation and reproductive timing. This makes it useful as part of herd fertility management under veterinary guidance.
Benefits
- Ovarian cyst treatment: Helps resolve non-ovulated follicles to encourage normal reproductive cycles in dairy cattle.
- Estrous synchronization: Works with cloprostenol sodium to align heat cycles for reliable breeding timing.
- Injection flexibility: Can be given intramuscularly or intravenously based on veterinarian instructions.
- Veterinary trusted: FDA approval and long-standing use in reproductive protocols make it a dependable choice.
- No milk or meat withdrawal: When used as directed, cows do not require milk discard or meat withholding periods.
Variants
10 mL vial
30 mL vial
100 mL vial
Ingredients
Active Ingredients
Gonadorelin diacetate tetrahydrate (equivalent to 43 mcg gonadorelin per mL)
Inactive Ingredients
Benzyl alcohol, Sodium chloride, Water for injection, Potassium phosphate (buffer)
How to Use
Recommended Dosage Chart
|
Indication |
Approved Labeled Dose |
Species |
|
Cystic ovaries |
100 mcg gonadorelin diacetate tetrahydrate (2 mL) |
Dairy cattle |
|
Estrous synchronization |
100 mcg gonadorelin diacetate tetrahydrate (2 mL) |
Dairy and beef cattle |
Dosage & Administration Instructions
- Labeled dosage: The product is approved at a labeled dose of 100 mcg gonadorelin per animal.
- Approved routes: Labeling states the solution is approved for intramuscular or intravenous administration only.
- Synchronization protocols: Use with cloprostenol sodium is labeled for estrous cycle synchronization in approved breeding programs.
- Veterinary oversight: Federal law restricts this drug to veterinary prescription use only.
Disclaimer: This dosage information is provided by the manufacturer. Always consult your veterinarian before administering or adjusting any supplement for your pet.
Additional Information
Precautions
- Human safety: The product is not intended for human use and should be handled accordingly.
- Prescription status: Use is restricted to licensed veterinarians under federal regulation.
- Child safety: Labeling advises keeping the product out of reach of children at all times.
Possible Side Effects
- Injection reactions: Field studies showed no significant increase in injection site reactions compared to placebo groups.
- Health abnormalities: Clinical trials reported no meaningful increase in health issues versus untreated cattle.
Storage Information
- Temperature limits: The solution is labeled for storage at or below 77°F with brief excursions permitted.
- After opening: The Manufacturer labeling states the vial should be used within six months of first puncture.
- Handling care: Store in original packaging to maintain sterility and product stability.
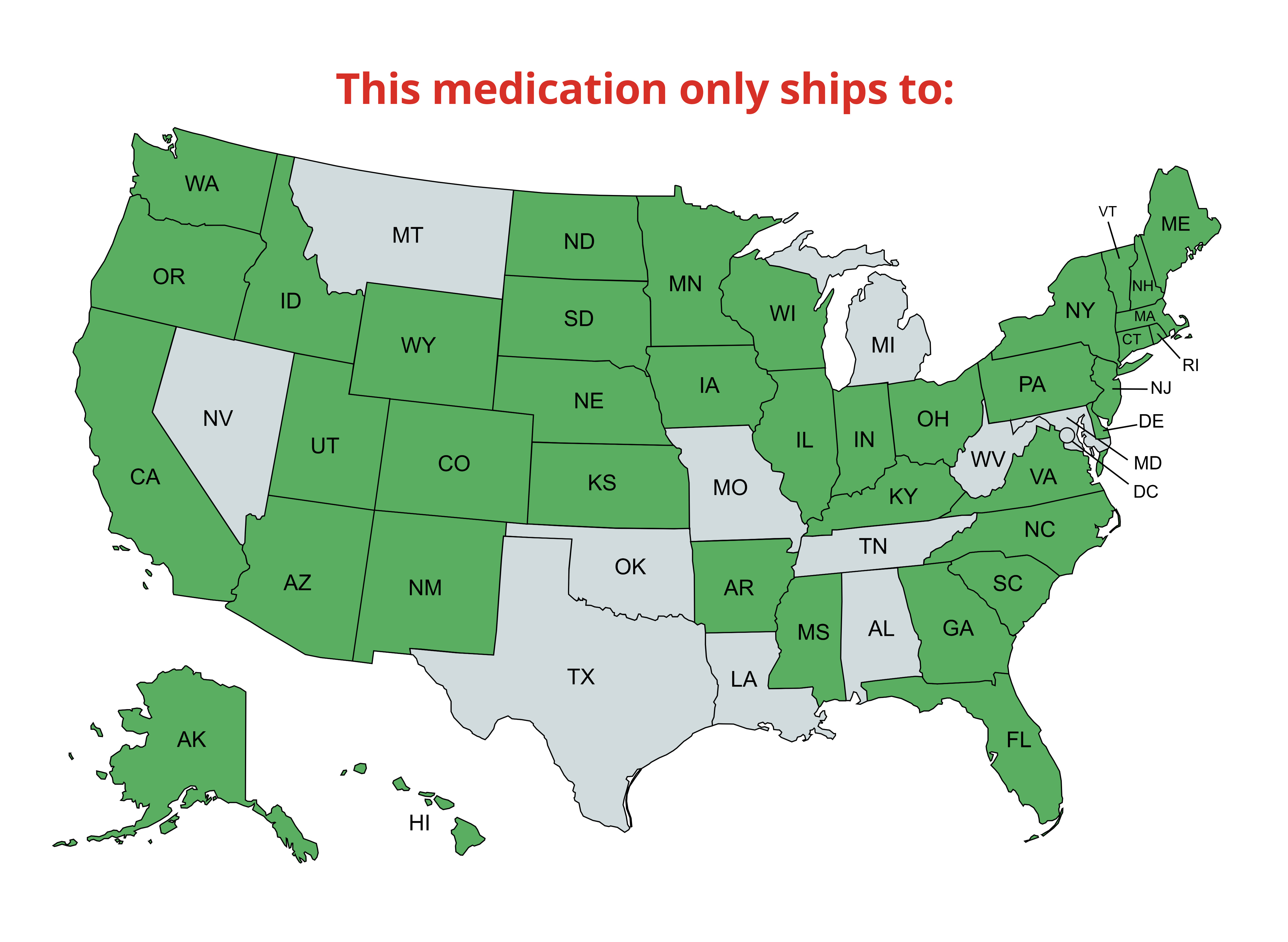
Shipping & Return
We offer ground, express, priority, and international delivery; see our shipping policy for details.
For return instructions or product concerns, please refer to our detailed refund policy.
Prescription items are NON-RETURNABLE and NON-REFUNDABLE.
Frequently Asked Questions
Q1: What is CYSTORELIN used for in cattle?
Ans: CYSTORELIN is FDA approved for treating ovarian follicular cysts in dairy cattle and for synchronizing estrous cycles in dairy and beef cows when used with cloprostenol sodium as part of fixed time artificial insemination programs under veterinary supervision.
Q2: Is CYSTORELIN safe for use in lactating cows?
Ans: According to manufacturer labeling and FDA review, CYSTORELIN has no required milk discard or meat withdrawal period when used as directed, making it suitable for lactating dairy cows under approved reproductive management protocols supervised by a licensed veterinarian.
Q3: Does CYSTORELIN require a veterinary prescription?
Ans: Yes, CYSTORELIN is a prescription only veterinary drug. Federal law restricts its use to administration by or on the order of a licensed veterinarian, ensuring proper oversight for reproductive hormone therapy in cattle.
Q4: How does CYSTORELIN support reproductive performance?
Ans: CYSTORELIN contains synthetic gonadorelin, which is identical to the natural hormone that stimulates release of luteinizing hormone and follicle-stimulating hormone, supporting ovulation, luteinization, and improved reproductive timing in cattle breeding programs.
Q5: What vial sizes are available for CYSTORELIN?
Ans: Manufacturer labeling states that CYSTORELIN is supplied in multi-dose sterile vials, including 10 mL, 30 mL, 50 mL, and 100 mL options, allowing veterinarians to select an appropriate size based on herd management needs.