Product title
Vendor
$19.99 | $24.99
Product title
Vendor
$19.99 | $24.99
Product title
Vendor
$19.99 | $24.99
Product title
Vendor
$19.99 | $24.99
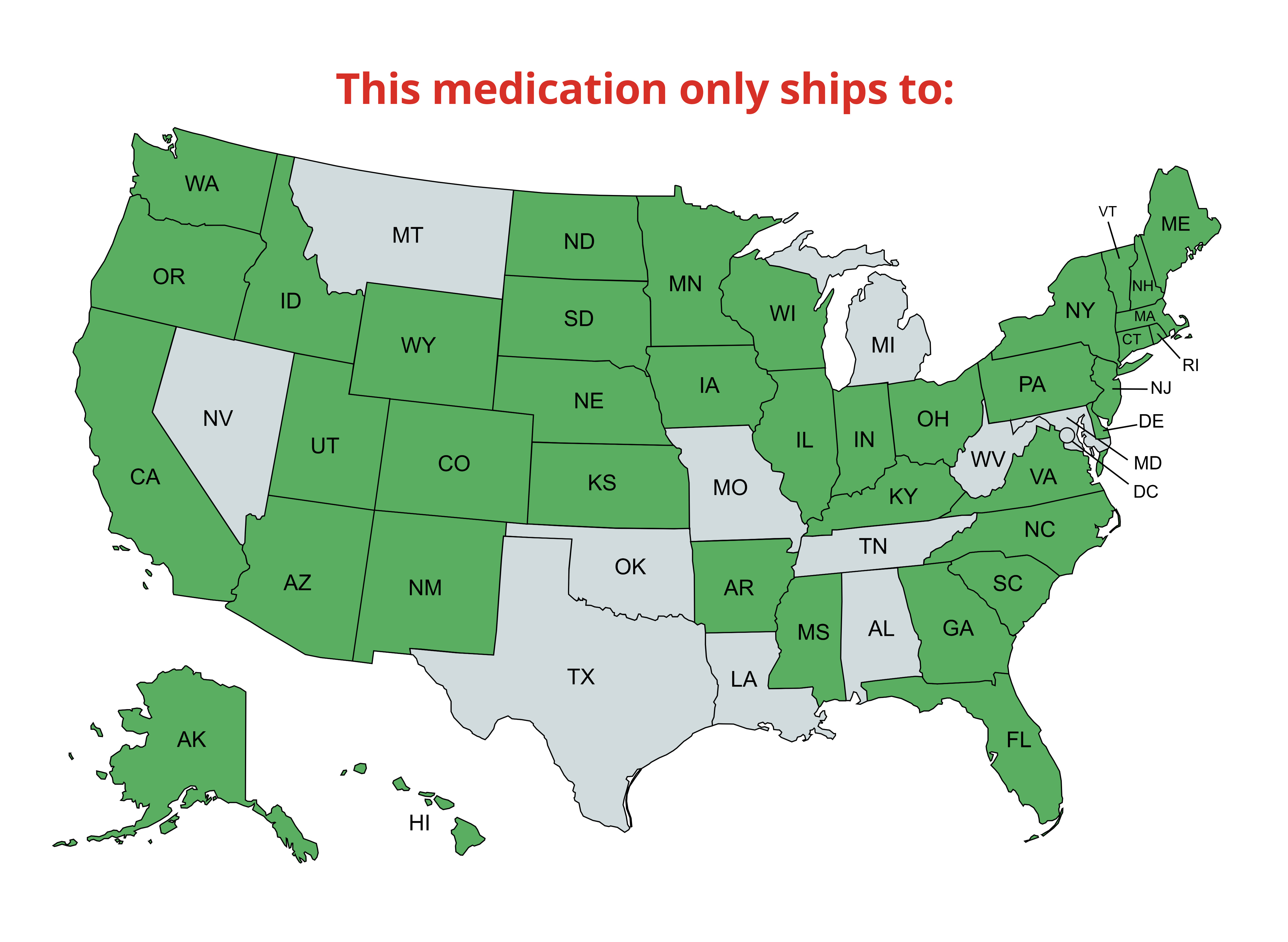
NOTICE: This item requires Express (1-2 day) shipping due to the perishable nature of this product. Express orders are shipped Monday-Wednesday.
OstiFen (Carprofen) Injection for Dogs
Ostifen Carprofen Injection is an advanced, fast-acting solution specifically formulated for dogs suffering from arthritis, post-surgical pain, and other inflammatory conditions. It is a non-steroidal anti-inflammatory drug (NSAID) from the propionic acid class, similar to ibuprofen, naproxen, and ketoprofen.
This injectable form of Carprofen delivers quicker and more precise relief, making it an ideal choice for veterinarians and pet owners looking for immediate and effective pain management. Ostifen significantly improves your dog’s mobility and quality of life, ensuring they can enjoy daily activities with minimal discomfort.
Prescription items are NON-RETURNABLE and NON-REFUNDABLE.
Please note that the product may arrive in a Hardy Paw Pharmacy vial, manufacturer packaging is shown for reference.
Benefits of Ostifen Carprofen Injection
- Rapid Onset of Action: Provides faster pain relief than oral alternatives, with effects noticeable within 1-2 hours post-injection.
- Precise Dosage: Ensures consistent and accurate dosing, reducing the likelihood of missed or partial doses that can occur with oral medications.
- Veterinary-Grade Quality: Trusted by veterinarians for its effectiveness and safety, ensuring reliable treatment outcomes.
- Versatile Usage: Suitable for both short-term post-operative pain and long-term management of chronic conditions like osteoarthritis.
- Enhanced Mobility: Helps reduce joint stiffness, allowing dogs to regain movement and enjoy a more active lifestyle.
- Reduced Frequency of Administration: Can be administered as a single daily dose, simplifying the treatment regimen for both pets and owners.
Subscription and Purchase Information
Buying Options and Discounts: Purchase Ostifen Carprofen Injection in a convenient 3-week, 1, 2, 3, and 6-month supply pack. Subscribe for regular shipments to ensure you never miss a dose and enjoy discounts on your purchases.
Order online for hassle-free delivery to your doorstep.
Ingredients
- Primary Ingredient: Carprofen (NSAID) - 50 mg/ml
- Secondary Ingredients: Benzyl alcohol, L-arginine, Water for Injection
How Does Ostifen Carprofen Injection Work?
Ostifen Carprofen Injection works by inhibiting the cyclooxygenase (COX) enzymes, specifically COX-2, which play a significant role in prostaglandin production. Prostaglandins are responsible for pain and inflammation. By reducing their production, Carprofen effectively diminishes inflammation, alleviates pain, and helps restore your dog’s comfort and mobility.
Dosage and Administration
- Carefully consider the potential benefits and risks of carprofen and other treatment options before using OstiFen Injection.
- Use the lowest effective dose for the shortest duration consistent with individual response.
- The recommended dosage for subcutaneous administration to dogs is 2 mg/lb (4.4 mg/kg) of body weight daily.
- The total daily dose may be administered as either 2 mg/lb of body weight once daily or divided and administered as 1 mg/lb (2.2 mg/kg) twice daily.
- For control of postoperative pain, administer approximately 2 hours before the procedure.
Frequency
Ostifen Carprofen Injection should be administered once daily.
Consistency
To achieve the best results, consistent administration is important. The full dose should be administered at the same time each day, especially when used for chronic conditions like osteoarthritis.
Side Effects of OstiFen Injection
While Ostifen Carprofen Injection is generally safe and well-tolerated, some dogs may experience side effects. Common side effects include:
- Gastrointestinal Issues:
- Vomiting
- Diarrhea
- Loss of appetite
- Abdominal pain
- Behavioral Changes:
- Lethargy
- Restlessness
- Increased thirst
- Renal (Kidney) Effects:
- Increased urination
- Signs of kidney dysfunction (e.g., changes in urine color or volume)
- Hepatic (Liver) Effects:
- Jaundice (yellowing of the skin, gums, or eyes)
- Elevated liver enzymes (detected via blood tests)
- Allergic Reactions:
- Hives
- Swelling (especially of the face, lips, or tongue)
- Difficulty breathing
- Neurological Issues:
- Dizziness or disorientation
- Unsteadiness or stumbling
If your dog experiences any of these side effects, discontinue use immediately and consult your veterinarian for further advice.
Precautions
- Undiagnosed Underlying Conditions: NSAID therapy could unmask previously undiagnosed conditions, such as renal disease, due to the absence of apparent clinical signs.
- Surgical Use: Consider using parenteral fluids during surgery to mitigate the risk of renal complications associated with NSAID use.
- Concomitant Medication Risks:
- Nephrotoxic Drugs: Use caution when administering Ostifen alongside other potentially nephrotoxic drugs. Monitoring is essential.
- Other NSAIDs and Corticosteroids: Avoid concurrent use due to the increased risk of gastrointestinal ulceration or other adverse reactions.
- Injection Safety:
- Hygienic Practices: Proper hygiene should be maintained during injection to prevent complications.
- Site Rotation: It’s recommended to use different sites for multiple injections.
Contraindications and Unestablished Safety
- Bleeding Disorders: Ostifen is not recommended for dogs with bleeding disorders, such as Von Willebrand's disease, due to unestablished safety.
- Young, Pregnant, or Breeding Dogs: Safety in puppies under 6 weeks of age, pregnant dogs, dogs used for breeding, or lactating bitches has not been established.
- Administration Route: Safety for intravenous (IV) or intramuscular (IM) administration has not been confirmed.
Drug Interactions
- Protein-Bound Drugs: Monitoring is necessary when administering Ostifen with other protein-bound or similarly metabolized drugs.
- Anesthetic Interaction: Carprofen may reduce the need for inhalant anesthetics during procedures.
- Pain Management: If additional pain relief is needed after Ostifen administration, alternative analgesics should be considered instead of another NSAID.
Warnings
- Not for use in cats.
- Discontinue if any signs of allergic reaction, such as hives or difficulty breathing, occur.
- Seek veterinary advice if your dog shows signs of gastrointestinal distress or behavioral changes.
Storage
- Store Ostifen Carprofen Injection in a cool, dry place away from direct sunlight.
- Keep it at a temperature between 20°C to 25°C (68°F to 77°F).
- Ensure that it is kept out of reach of children and pets.
- Do not use after the expiration date printed on the packaging.
Shipping & Returns
Shipping Policy: We offer all types of shipping options (Ground, Express, Priority, International) depending on the urgency of the product requirements. To know about our shipping policy in detail, click here.
Refund policy: If you face any issue with our product and want to return it, read our refund policy carefully.
FAQs - OstiFen Injection for Dogs
Q: What is Ostifen Carprofen Injection used for?
A: Ostifen Carprofen Injection is used to treat pain and inflammation in dogs, particularly associated with osteoarthritis, post-surgical recovery, and other musculoskeletal disorders. It provides fast-acting relief by reducing inflammation, improving mobility, and helping dogs recover more comfortably from surgeries or manage chronic pain conditions effectively.
Q: Can Ostifen Carprofen Injection be used with other pain medications?
A: Always consult your veterinarian before combining Ostifen with other medications, as drug interactions could occur, potentially leading to adverse effects.
Q: How long can my dog stay on Ostifen Carprofen Injection?
A: Ostifen can be used for both short-term and long-term pain management. However, long-term use requires regular veterinary monitoring to avoid potential side effects.
Q: Is there a risk of dependency or tolerance with long-term use of Ostifen?
A: Dogs do not develop dependency or tolerance to NSAIDs like Carprofen. However, long-term use may require periodic dose adjustments based on your dog’s condition and response to treatment.
Q: Can I switch from Ostifen injection to oral Carprofen tablets?
A: Yes, switching from injection to oral tablets is possible, but dosage adjustments may be necessary. Consult your veterinarian for the correct transition protocol.
Q: What should I do if my dog shows signs of an allergic reaction to Ostifen?
A: Discontinue use immediately and seek emergency veterinary care if your dog exhibits signs of an allergic reaction, such as hives, swelling, or difficulty breathing.
Q: How does Ostifen compare to other NSAIDs on the market?
A: Ostifen is comparable in efficacy to other NSAIDs but offers faster pain relief due to its injectable form, making it ideal for acute pain management.
Q: Can I administer Ostifen Carprofen Injection at home?
A: While Ostifen is typically administered by a veterinarian, some pet owners may be trained to give the injection at home. Always follow your vet’s instructions closely.
Q: Does Ostifen Carprofen Injection require refrigeration?
A: No, Ostifen does not require refrigeration. It should be stored at room temperature, away from direct sunlight.
Q: How do I know if Ostifen is working for my dog?
A: Signs that Ostifen is effective include reduced pain behaviors, improved mobility, and an increase in your dog’s activity levels. Regular veterinary check-ups can also confirm its effectiveness.
Q: Is there a generic alternative to Ostifen Carprofen Injection?
A: Yes, there are generic versions of Carprofen available, but it’s essential to consult your veterinarian before switching to ensure it’s appropriate for your dog’s condition.
Clinical Research:
https://www.sciencedirect.com/science/article/pii/S1467298721000945


